The National Agency for Food and Drug Administration and Control (NAFDAC) said it has detected suspected falsified Augmentin 625mg tablets in circulation within the country.
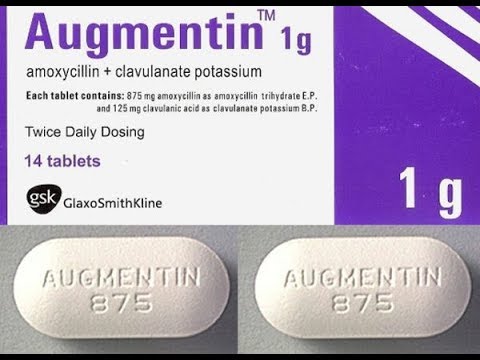
In a message titled, ‘Alert and Sensitisation on Suspected Falsified Augmentin 625MG’ NAFDAC listed the following details: Augmentin 625mg; Batch No.: 562626; manufacturing date: April 2021; expiry date: April 2024; NAFDAC Reg No: 04-1928.
It also listed the following as labelling lapses;
The product failed short of the labeling requirements.
No inscription “manufactured by” is written on the label -only the address.
Manufacturing and Expiry dates do not meet the acceptable format.
No MAS scratch number for verification.
The logo “gsk” is not properly positioned as on the original.
The listed information indicates the product is falsified and counterfeited.
In view of the above, NAFDAC has notified all its formations in the zones and 36 states of the federation including the FCT to carry out surveillance and mop up the falsified Augmentin tablets.
Please note that the genuine Augmentin 625mg has legible product labelling information including date markings – expiration and manufactured dates, batch number and NAFDAC registration number.
Members of the public in possession of the above suspected counterfeit product are advised to discontinue sale or use and submit stock to the nearest NAFDAC office.
Report any suspicion of adverse drug reaction and substandard and falsified medicines to NAFDAC on 0800-162-3322 or sf.alert@nafdac.gov.ng
(SolaceBase)